
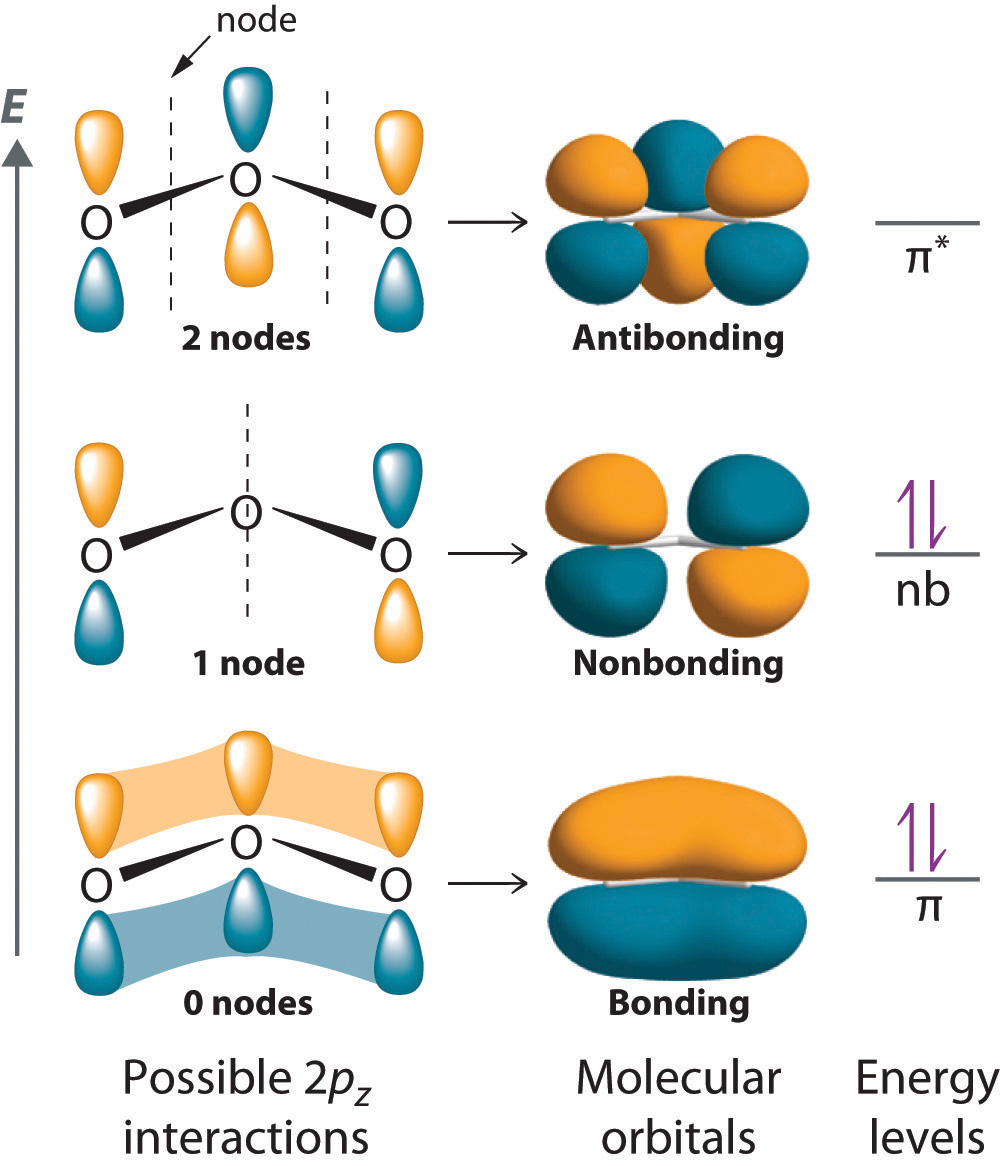
Orbitals of all types are simply mathematical functions that describe particular standing-wave patterns that can be plotted on a graph but have no physical reality of their own. The quantity that is varying ( waving) is a number denoted by ψ ( psi) whose value varies from point to point according to the wave function for that particular orbital. Because of the wavelike character of matter, the orbital corresponds to a standing wave pattern in three-dimensional space which we can often represent more clearly in two-dimensional cross Will defer a discussion of it until a later unit.įor now, we will look at a less-radical model that starts out with the familiar valence-shell atomic orbitals, and allows them to combine to form hybrid orbitals whose shapes conform quite well to the bonding geometry that we observeįirst, recall that the electron, being a quantum particle, cannot have a distinct location the most we can do is define the region of space around the nucleus in which the probability of finding the electron exceeds some arbitrary value, such as 90% In fact, as far as valence electronsĪre concerned, we can throw out the concept of atomic orbital altogether and reassign the electrons to a new set of molecular orbitals that are characteristic of each molecular configuration.
#Hybridization of atomic orbitals free
An outer-shell electron in a bonded atom willīe under the influence of a force field emanating from two positive nuclei, so we would expect the orbitals in the bonded atoms to have a somewhat different character from those in free atoms. Remember that these different orbitals arise in the first place from the interaction of the electron with the single central electrostatic force field associated with the positive nucleus. These facts suggest that it is incorrect to assume that the distribution of valence electrons that are shared with other atoms can be described by atomic-type s, p, and d orbitals at all. In the two bonds shared Be orbitals of different types, as in the excited state diagram above.

Moreover, the two bonds in BeH 2 and similar molecules are completely equivalent this would not be the case if the electrons It is observed that Be does form reasonably stable bonds with other atoms. However, the energy required to produce this excited-state atom would be sufficiently The only way that we can obtain two unpaired electrons for bonding in beryllium is to promote one of the 2 s electrons to the 2 p level. Note that the two electrons in the 2 s orbital have opposite spins and constitute a stable pair that has no tendency to interact with unpaired electrons on other atoms. The beryllium atom, with only four electrons, has a configuration of 1 s 22 s 2. Why Atomic Orbitals Do Not Work for MoleculesĬonsider how we might explain the bonding in a compound of divalent beryllium, such as beryllium hydride, BeH 2. As we shall see below, his assumption very quickly leads us into difficulties. As useful and appealing as the concept of the shared-electron pair bond is, it raises a somewhat troubling question that we must sooner or later face: what is the nature of the orbitals in which the shared electrons areĬontained? Up until now, we have been tacitly assuming that each valence electron occupies the same kind of atomic orbital as it did in the isolated atom.


 0 kommentar(er)
0 kommentar(er)
